Luye Pharma is an international leader in advanced drug delivery systems such as microspheres, liposomes and transdermal drug delivery. The company has also achieved multiple innovations on its technical platforms for new chemical entities and antibodies, and is actively working in areas such as cell therapy and gene therapy.
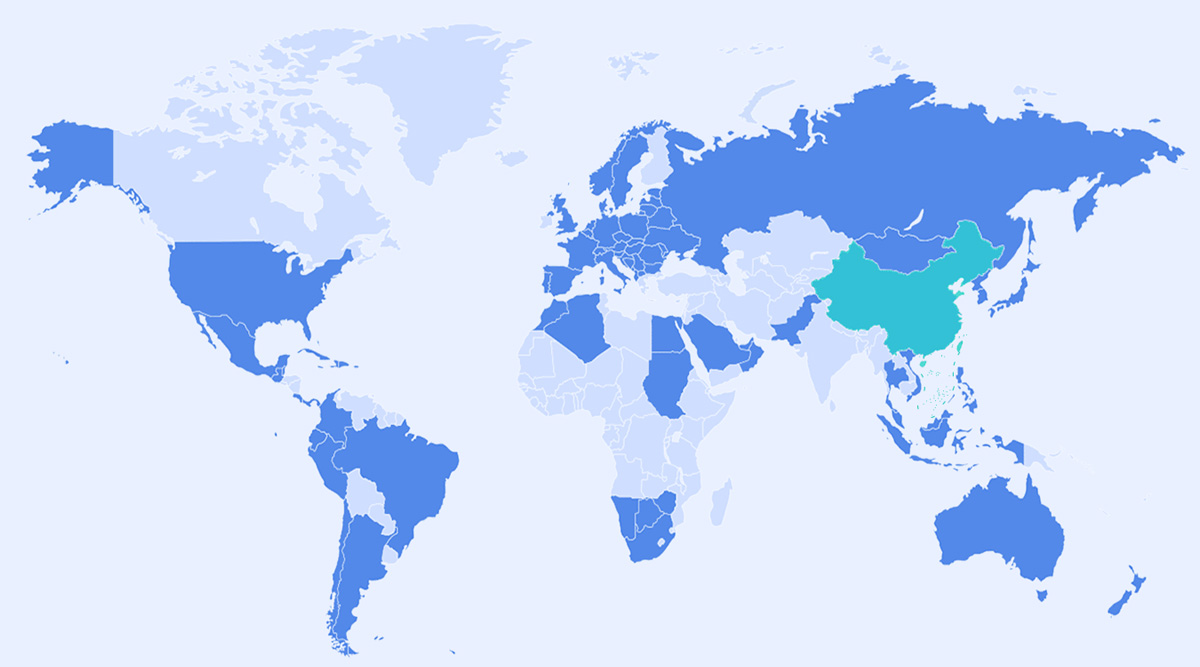
 China
China
Key R&D Capabilities:long-acting and extended release technology, liposome and targeted drug delivery technology, NME platforms,biological antibody technology, innovative medical technology
 U.S.A
U.S.A
Key R&D Capabilities: international R&D, collaboration exploratory study for innovative drugs.
 Europe
Europe
Key R&D Capabilities: Transdermal Drug Delivery Technology
Novel Drug Delivery Systems(NDDS)
NME (New Molecular Entity) Platforms

Human Antibody Transgenic Mouse and Phage Display Technology
Bispecific T-cell Engager Technology
Antibody Drug Conjugate Technology

Nucleic Acid Therapy and Gene Therapy
Gene and Nucleic Acid Targeted Delivery
CAR-T Cell Immunotherap
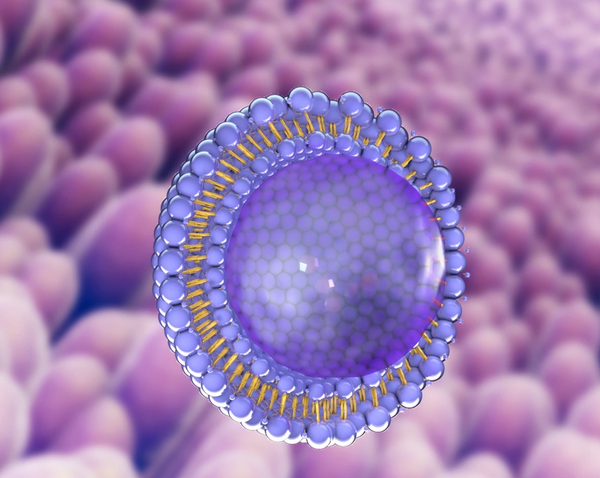
A liposome is a miniscule bubble (vesicle) made out of similar material to a human cell membrane that can be filed with active pharmaceutical ingredients, including macromolecules such as proteins and peptides Liposomes can vary in size and the number of bilayers,
Microsphere technology utilises biodegradable polymer micrometre-sized particles that can encapsulate many types of drugs, including small molecules, proteins and nucleic acids, and are easily administered through injections Administered through subcutaneous or intramuscular injections, these formulations are designed for sustained release over a predetermined period of time from half a month to longer than one month
Transdermal system is kind of therapeutic drug for disease treatment or prevention, through which, drug was absorbed to whole blood circulation by vessels of dermis from epidermis and reach to the effective blood concentration
Luye Pharma has 8 manufacturing sites with 30+ production lines. 7 manufacturing sites locate in Yantai, Nanjing, Beijing, Luzhou and Chengdu of China, 1 manufacturing site locates in Germany.

Passed EU GMP inspections
Passed Australia TGA GMP inspections
Passed Brazilian ANVISA inspections
Passed China 2010 GMP inspections
Obtained ISO9001:2008 QMS certification
Obtained ISO14001:2015 EMS certification
Obtained OHSAS18001:2007 certification
Obtained CNAS certification

Passed EU GMP inspections
Passed FDA cGMP inspections
Passed Japanese GMP inspections
Passed Brazilian ANVISA inspections
Passed Columbian INVIMA inspections

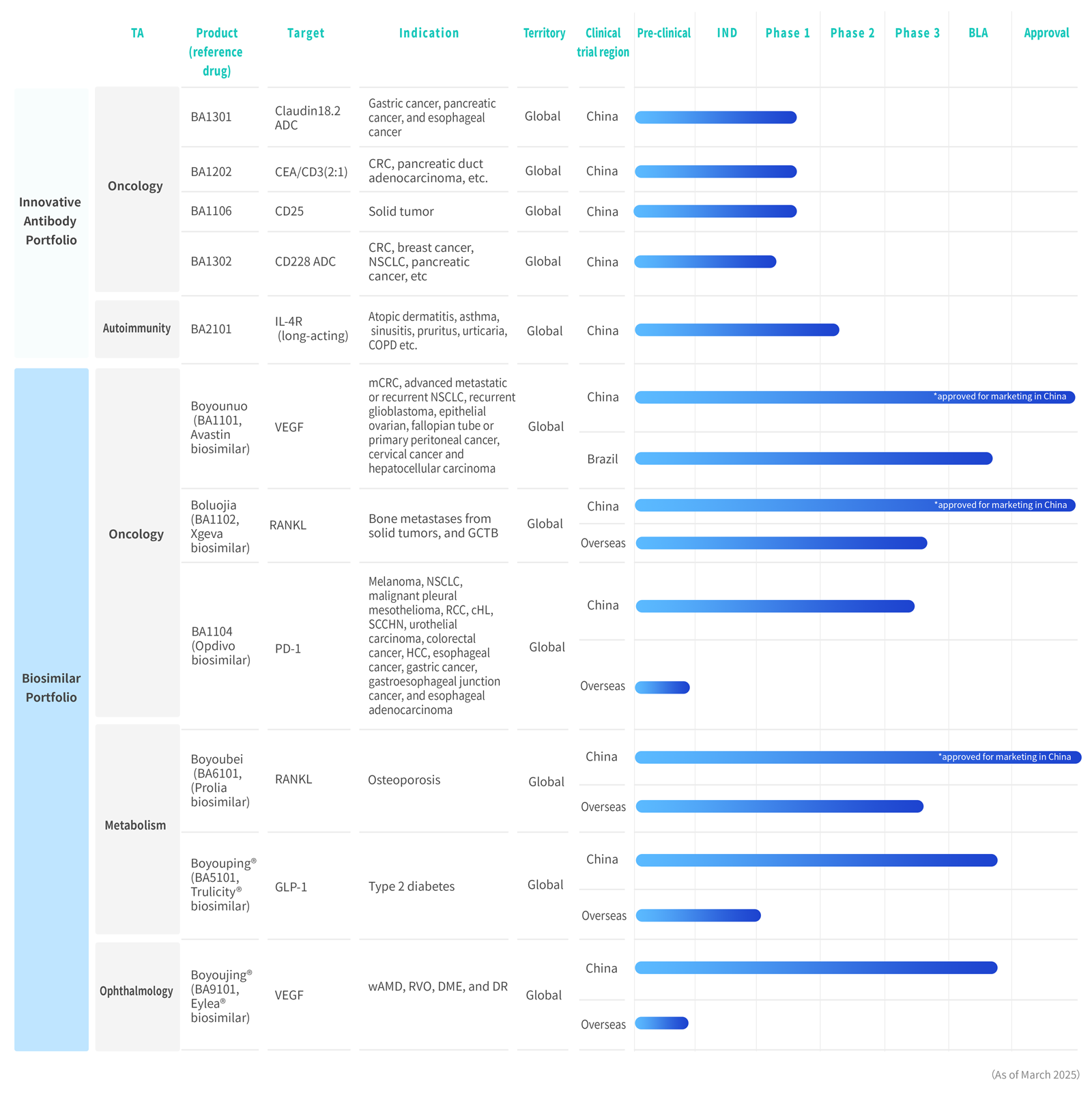
Aflibercept intravitreous injection,a biosimilar to EYLEA®

China’s First Locally Developed Dulaglutide Injection

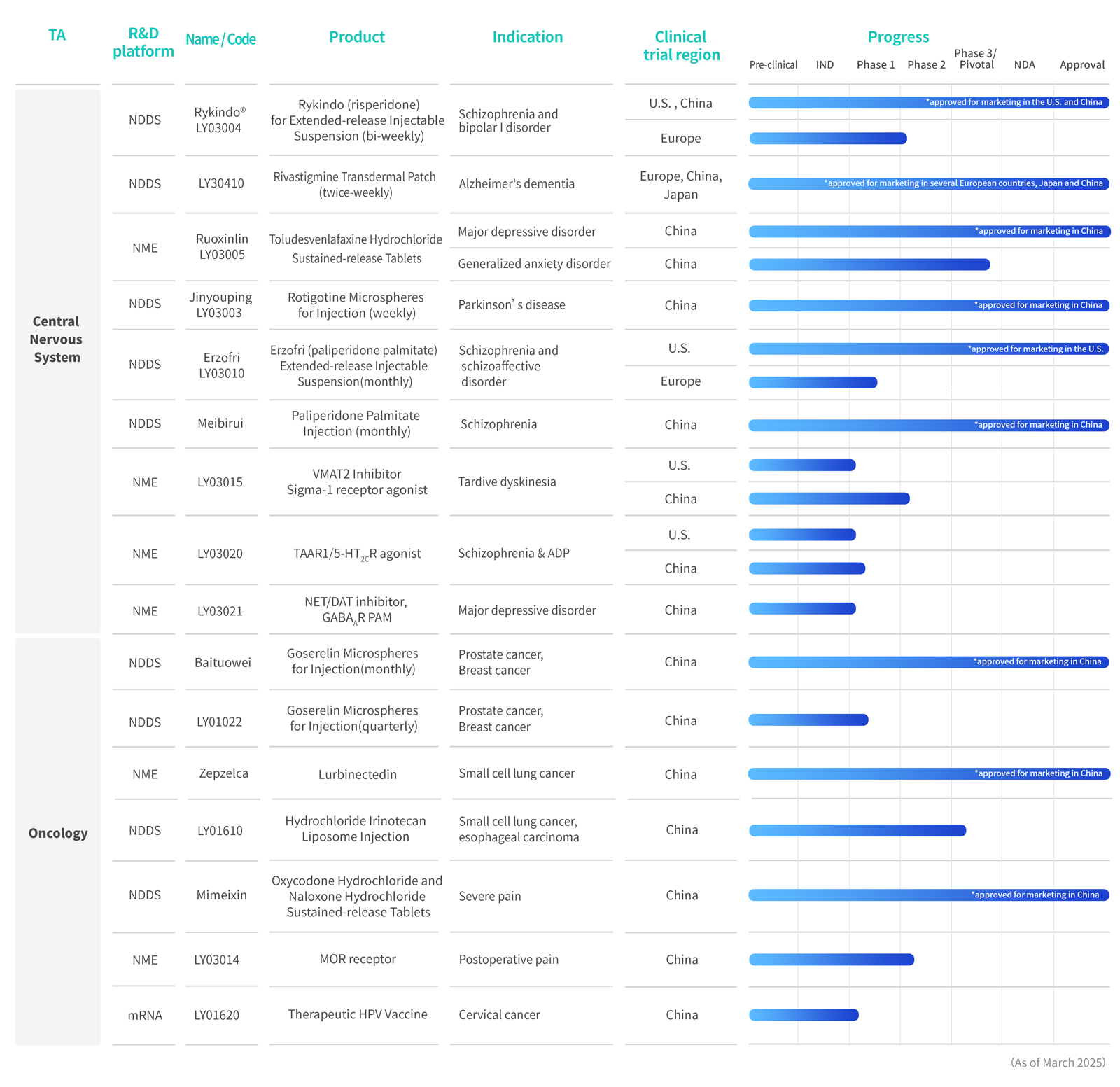
Approved as a new drug for the treatment of schizophrenia and schizoaffective disorder; administered once per month

The only locally made product of oxycodone hydrochloride and naloxone hydrochloride sustained-release tablets in China

Paliperidone palmitate injection

The world’s first innovative microsphere formulation for treating Parkinson’s disease

The world's only formulation of long-acting goserelin microspheres approved for launch in China

The only new chemical entity approved by the FDA for the treatment of metastatic SCLC in the past 26 years

For the treatment of postmenopausal women with osteoporosis at high risk of fracture

Toludesvenlafaxine Hydrochloride Extended-Release Tablets, for treating Major Depressive Disorder (MDD)

Chemical sensitizer for cancer radiotherapy

Received marketing approval in China for the treatment of NSCLC, colorectal cancer, glioblastoma, ovarian cancer and cervical cancer.

Independently developed innovative microsphere formulation, for treatment of schizophrenia

Atypical anti-psychotic medicines with antidepressant properties

The paclitaxel liposome injection indicated for multiple cancers

Xuezhikang—The evidence-based and internationally recognized Chinese lipid-adjusting drug

Received marketing approval in several European countries for the treatment of mild to moderate dementia associated with Alzheimer's disease
A commercial operation centered at the Group level, with products covering approximately 21,000 hospitals across 31 provinces, municipalities and autonomous regions in China. From among these, oncology products are sold to 3,000 hospitals and CNS products sold to 3,000 hospitals
Marketing network covers over 80 countries and regions including large global pharmaceutical markets and fast growing emerging markets. Both B-to-B and B-to-C business models are adopted.