
Luye Pharma Group announced that a research paper on LY-CovMab, an innovative antibody developed by Boan Biotech, a holding subsidiary of the Group, has been published in Communications Biology, a sub-journal of Nature magazine.

The COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) poses a tremendous threat to human health and the global economy. LY-CovMab is a human monoclonal neutralizing antibody that binds specifically to the receptor-binding domain in SARS-CoV-2 Spike protein with high affinity, and can effectively block the attachment of the virus to the host cell surface receptor ACE2.
The research demonstrates that LY-CovMab exhibits excellent neutralization potency against pseudovirus and authentic virus infection.
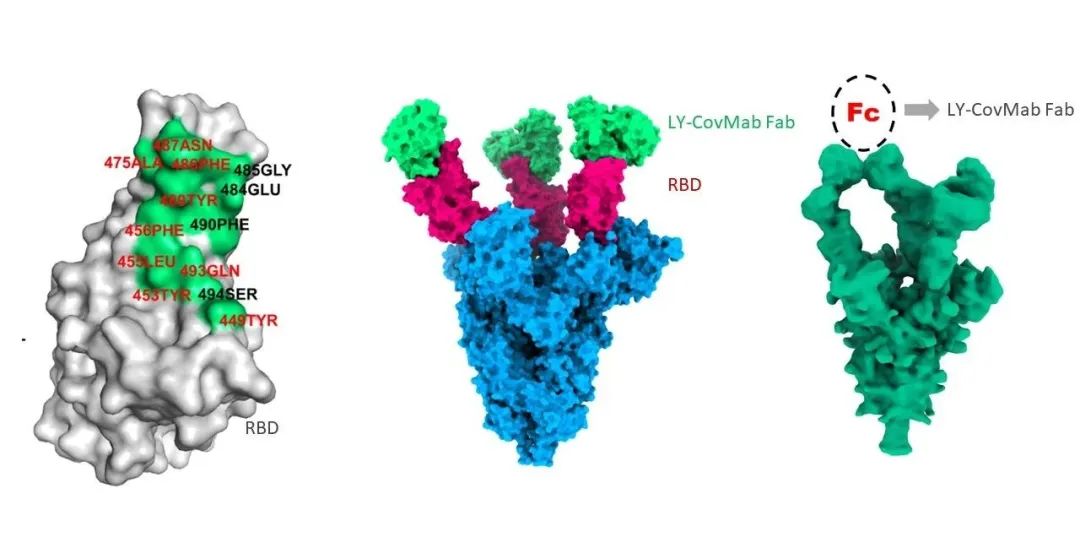
▲Structural Analysis of LY-CovMab by Cryo-EM
Dr. Changlin Dou, the corresponding author, R&D President and Chief Operating Officer of Boan Biotech said:
"These results demonstrate the importance of antibody-based therapeutic interventions against COVID-19 and identify CA521FALA as a promising antibody that reacts with SARS-CoV-2 Spike protein to strongly neutralize its activity. Boan Biotech has also conducted additional variants studies and found that LY-CovMab has good neutralization potency against SARS-CoV-2 variant B.1.1.7, which derives from the UK and occurs at a relatively higher frequency."
Boan Biotech is one of the first innovative biopharmaceutical companies in China to develop neutralizing antibodies against SARS-CoV-2. In developing this candidate, Boan Biotech has shown the efficiency of its antibody development capabilities via its human antibody transgenic mouse technology and phage display technology. BA-huMab are the first human antibody transgenic mice in China and have been used in multiple programs. Antibodies derived from BA-huMab do not require the antibody humanization process. Boan Biotech was able to obtain the lead antibody against SARS-CoV-2 in less than 50 days using this platform. In addition, LY-CovMab adopted a special design for the Fc region, which reduced the ADE risk and improved the safety profile of the antibody.
Currently, LY-CovMab is at the late stage of the phase I clinical trial in China. China’s Ministry of Science and Technology has included LY-CovMab in its COVID-19 Neutralizing Antibody Emergency Projects. Outside of China, Boan Biotech will also carry out clinical research with patients in the United States to support the launch of LY-CovMab in global markets.

